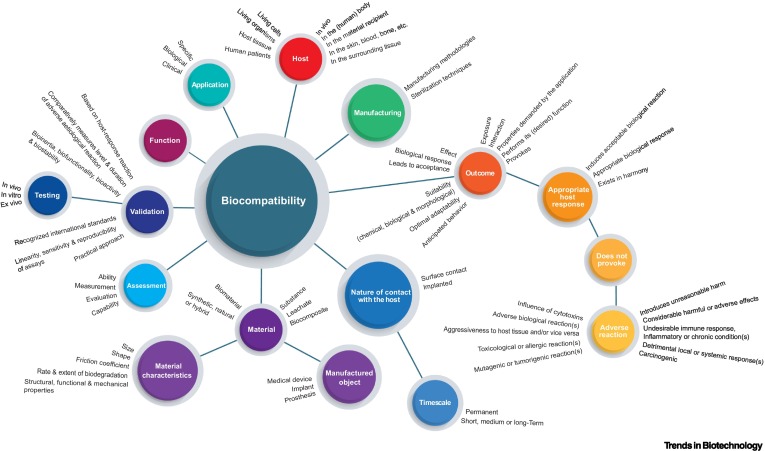
Biocompatibility, the ability of a material to perform its intended function within the body without causing harm, has been a central concern in biomaterials research. However, defining and assessing biocompatibility has posed substantial challenges due to the diverse nature of biomaterials and the intricacies of their interactions within the human body.
The study, conceptualised by Dr Athina Samara, explores the need for a new definition of biocompatibility that includes AI-driven text mining and highlights the challenges to scale biocompatibility. They emphasise on the difficulties posed by the vast, heterogeneous, and often inconsistent data related to biocompatibility. This variability stems from differences in experimental designs, parameters, and the sheer volume of data generated by various studies.
One of the critical insights of the research is the absence of structured databases for biocompatibility data in biomaterials, which has hindered progress. To address this challenge, the authors propose using automated tools like machine learning (ML) and artificial intelligence (AI) to extract valuable information from text and compile datasets. These tools can identify hidden patterns and swiftly decipher complex data, accelerating innovation and knowledge advancement.
The paper also highlights the power of natural language processing (NLP) techniques and large language models in extracting information from text and suggests their potential application in biomaterials research. However, it underscores the need for a consensus on the definition of biocompatibility to utilise these technologies effectively.
Dr Athina Samara and her BIOMATDB team reviewed existing definitions of biocompatibility from various sources, including international standards, and identified their strengths and limitations. They emphasise that biocompatibility should not only focus on safety but also consider functionality and other factors such as material properties, the interface with biological systems, anatomical location, and exposure time.
Based on their analysis, the researchers propose a comprehensive working definition of biocompatibility that takes these essential elements into account:
"Biocompatibility is the set of attributes describing the capability of a material to perform its desired function for the projected period without causing significant risk of local or systemic adverse response, irritation, toxicity, or any other adverse event in the recipient. The degree of biocompatibility depends on the material properties, the interface with the biological system, anatomical location, and the application duration or exposure time.", says Samara.
This new definition aims to provide clarity and structure for assessing biocompatibility. It could be a foundation for developing tools and databases for information extraction in biomaterials research.
The study acknowledges that while this proposed definition is a significant step forward, it raises several outstanding questions. These questions challenge the scientific community to further refine and standardise biocompatibility criteria, enhance testing protocols, and integrate clinical data into the definition.
Highlights
- Current definitions of biocompatibility lack clarity and consensus, making data extraction challenging for biomaterial evaluation.
- Establishing a working definition of biocompatibility is crucial for enabling computational tools to extract and analyse relevant information.
- The analysis of international standards has informed the development of a comprehensive working definition that includes relevant specifications and vocabulary for text mining.
- Identifying key elements and gaps in existing biocompatibility definitions has led to a unified and implementable working definition suitable for automated data extraction.
For more details on this research and its implications, you can access the full paper here
Acknowledgements
The authors would like to acknowledge funding from BiomatDB+ (Horizon Europe 101058779). Osnat received the EU-funded Marie Skłodowska-Curie Actions (MSCA) fellowship that led to the DEBBIE project. The funding bodies played no role in the study design, data collection, data analyses, interpretation, or writing.
